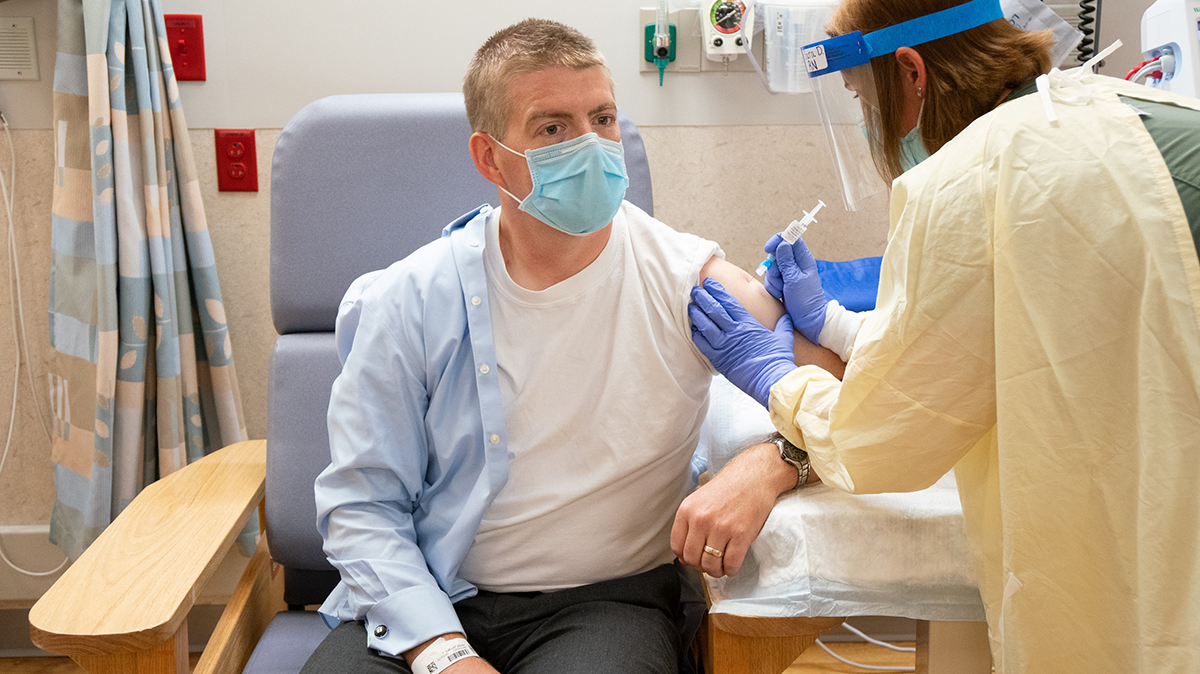
27 Oct AstraZeneca COVID-19 vaccine clinical trial resumes at UW
AstraZeneca announced that the COVID-19 vaccine trial has resumed in the United States, including at UW Health and the UW School of Medicine and Public Health, after the FDA and an independent safety review board completed their review of an illness contracted by a trial participant in the United Kingdom....